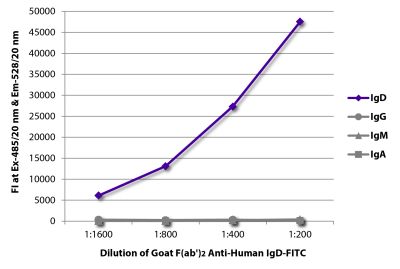
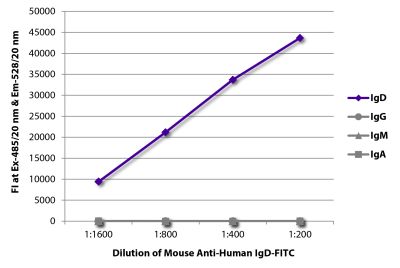
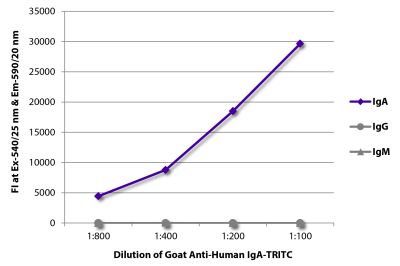
Goat Anti-Human IgD-TRITC
Cat. No.:
2030-03
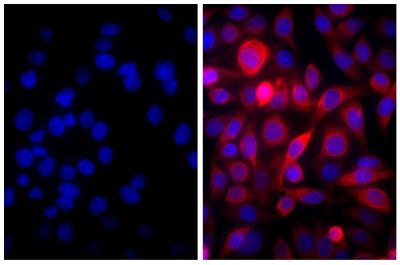
Goat Anti-Human IgD-TRITC antibody for use in immunohistochemistry assays.
$127.00
| Isotype | Goat IgG |
|---|---|
| Isotype Control | Goat IgG-TRITC |
| Specificity | Reacts with the heavy chain of human IgD |
| Source | Pooled antisera from goats hyperimmunized with human IgD |
| Cross Adsorption | Human IgG, IgM, and IgA; may react with IgD from other species |
| Purification Method | Affinity chromatography on human IgD covalently linked to agarose |
| Conjugate | TRITC (Rhodamine) |
| Buffer Formulation | Phosphate buffered saline containing < 0.1% sodium azide |
| Clonality | Polyclonal |
| Concentration | 1.0 mg/mL |
| Volume | 1.0 mL |
| Recommended Storage | 2-8°C; Avoid exposure to light |
| Applications |
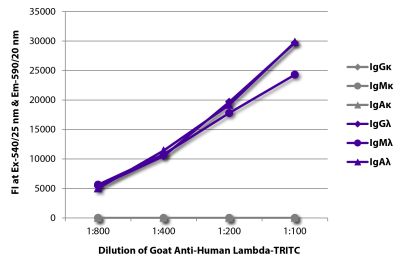
Quality tested applications for relevant formats include - ELISA FLISA Flow Cytometry 5-17 Other referenced applications for relevant formats include - Immunohistochemistry-Frozen Sections 1-4 Immunohistochemistry-Paraffin Sections 19 Separation 18 |
| RRID Number | AB_2795625 |
| Gene ID |
3495 (Human) |
| Gene ID Symbol |
IGHD (Human) |
| UniProt ID |
P01880 (Human |
| UniProt Name |
IGHD_HUMAN (Human) |
Documentation
Certificate of Analysis Lookup
Enter the Catalog Number and Lot Number for the Certificate of Analysis you wish to view
- 1. Pospisil R, Silverman GJ, Marti GE, Aruffo A, Bowen MA, Mage RG. CD5 is a potential selecting ligand for B-cell surface immunoglobulin: a possible role in maintenance and selective expansion of normal and malignant B cells. Leuk Lymphoma. 2000;36:353-65. (IHC-FS)
- 2. Hirbod T, Kaldensjö T, Broliden K. In situ distribution of HIV-binding CCR5 and C-type lectin receptors in the human endocervical mucosa. PLoS One. 2011;6(9):e25551. (IHC-FS)
- 3. Rakhmanov M, Sic H, Kienzler A, Fischer B, Rizzi M, Seidl M, et al. High levels of SOX5 decrease proliferative capacity of human B cells, but permit plasmablast differentiation. PLoS One. 2014;9(6):e100328. (IHC-FS)
- 4. Cols M, Barra CM, He B, Puga I, Xu W, Chiu A, et al. Stromal endothelial cells establish a bidirectional crosstalk with chronic lymphocytic leukemia cells through the TNF-related factors BAFF, APRIL, and CD40L. J Immunol. 2012;188:6071-83. (IHC-FS)
- 5. Mongini PK, Blessinger C, Posnett DN, Rudich SM. Membrane IgD and membrane IgM differ in capacity to transduce inhibitory signals within the same human B cell clonal populations. J Immunol. 1989;143:1565-74. (FC)
- 6. Kumagai M, Coustan-Smith E, Murray DJ, Silvennoinen O, Murti KG, Evans WE, et al. Ligation of CD38 suppresses human B lymphopoiesis. J Exp Med. 1995;181:1101-10. (FC)
- 7. Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679-89. (FC)
- 8. van der Loo JC, Hanenberg H, Cooper RJ, Luo F, Lazaridis EN, Williams DA. Nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse as a model system to study the engraftment and mobilization of human peripheral blood stem cells. Blood. 1998;92:2556-70. (FC)
- 9. Bräuninger A, Goossens T, Rajewsky K, Küppers R. Regulation of immunoglobulin light chain gene rearrangements during early B cell development in the human. Eur J Immunol. 2001;31:3631-7. (FC)
- 10. Fournillier A, Freida D, Defrance T, Merle P, Trépo C, Inchauspé G. Analysis of B-lymphocyte differentiation in patients infected with hepatitis C virus. J Med Virol. 2004;72:566-74. (FC)
- 11. Johansen F, Baekkevold ES, Carlsen HS, Farstad IN, Soler D, Brandtzaeg P. Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. Blood. 2005;106:593-600. (FC)
- 12. Dryer RL, Covey LR. Use of chromatin immunoprecipitation (ChIP) to detect transcription factor binding to highly homologous promoters in chromatin isolated from unstimulated and activated primary human B cells. Biol Proced Online. 2006;8:44-54. (FC)
- 13. Sira MM, Yoshida T, Takeuchi M, Kashiwayama Y, Futatani T, Kanegane H, et al. A novel immunoregulatory protein in human colostrum, syntenin-1, for promoting the development of IgA-producing cells from cord blood B cells. Int Immunol. 2009;21:1013-23. (FC)
- 14. Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281-94. (FC)
- 15. Pallikkuth S, Micci L, Ende ZS, Iriele RI, Cervasi B, Lawson B, et al. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS One. 2013;9(7):e1003471. (FC)
- 16. Yammani RD, Haas KM. Primate B-1 cells generate antigen-specific B cell responses to T cell-independent type 2 antigens. J Immunol. 2013;190:3100-8. (FC)
- 17. Neumann B, Klippert A, Raue K, Sopper S, Stahl-Hennig C. Characterization of B and plasma cells in blood, bone marrow, and secondary lymphoid organs of rhesus macaques by multicolor flow cytometry. J Leukoc Biol. 2015;97:19-30. (FC)
- 18. Joseph AM, Babcock GJ, Thorley-Lawson DA. Cells expressing the Epstein-Barr virus growth program are present in and restricted to the naive B-cell subset of healthy tonsils. J Virol. 2000;74:9964-71. (Sep)
- 19. SouthernBiotech unpublished data
See All References